Abstract
Introduction: Adolescent and Young Adult (AYA) patients with cancer are known to experience disparities in access to care along with delays in diagnosis and treatment. AYA patients with acute lymphoblastic leukemia (ALL) have poorer outcomes as compared to children with ALL. As a result, AYA patients with ALL are increasingly being treated in children's hospitals on pediatric protocols. However, minimal data exist regarding how well AYA patients tolerate the intensity of chemotherapy at doses and regimens designed for children. Our study aims to compare toxicities between pediatric and AYA populations treated for ALL in children's hospitals in the United States.
Methods: With IRB approval, the Pediatric Health Information Systems (PHIS) database was queried to analyze healthcare outcomes in pediatric and AYA patients with ALL (ICD-9 code 204.00) admitted between January 1999 and December 2014. We chose to utilize the PHIS database, as these AYA patients with ALL were more likely to have been treated on pediatric protocols. AYA was defined as patients between the ages of 15 and 39 according to the National Cancer Institute (NCI) definition. We extracted relevant ICD-9 diagnoses and procedure codes for each patient related to grades 3 or 4 toxicities as outlined by the NCI, including: infections, cardiac, gastrointestinal, neurological, pulmonary, psychological, and renal complications, thrombosis, avascular necrosis, sepsis, and procedures performed. We focused primarily on various drug-related toxicities specific to therapeutic agents used in pediatric protocols. Admission age, sex, race, ICU stay, and mortality were also analyzed. Nominal data were described with percents and continuous data with medians . Nominal variables were compared using Chi squared analysis; continuous variables were compared using Wilcoxon rank sum tests. All statistical analyses were performed using SAS v. 9.4 (SAS Institute, Cary, NC).
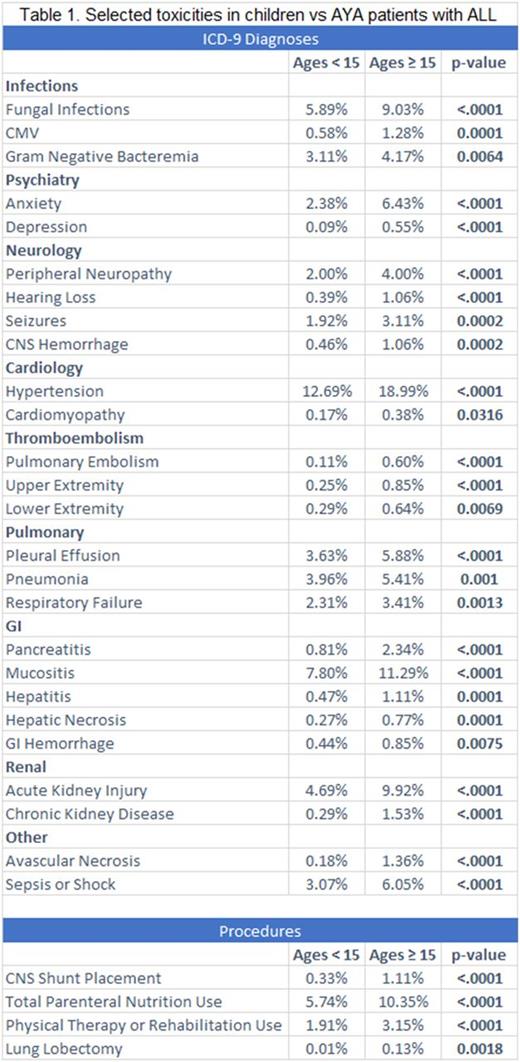
Results: A total of 18,095 admissions met inclusion criteria, representing 13,720 patients. Of these, 2,348 admissions (13.0%) were in the AYA age group. 10,896 patients (79%) had one admission and the remaining 2,824 (21%) had two or more admissions. 55% of children and 66% of AYA patients were male. AYA patients had a significantly higher incidence of ICU stay [16.3% vs 13.3%; p<0.0001], longer median length of hospital stay [9.0 vs 8.0 days; p=0.002], and increased mortality [3.9% vs 1.6%; p<0.0001]. The following table (Table 1) describes our key findings. The latter show statistically significant differences among the two groups, with AYA patients showing markedly increased toxicities.
Conclusions: In this large multicenter database study, we found overall increased number of toxicities among AYA patients with ALL. Compared to children, AYA patients developed disproportionately higher toxicities from drugs commonly used in pediatric protocols for ALL. Prospective studies are needed to assess whether dose modifications for certain chemotherapeutics may improve the toxicity profile and health care burden of AYA patients with ALL treated in children's hospitals.
Ahuja: Shire: Speakers Bureau; Shire: Honoraria; CSL Behring: Honoraria; Bayer: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal